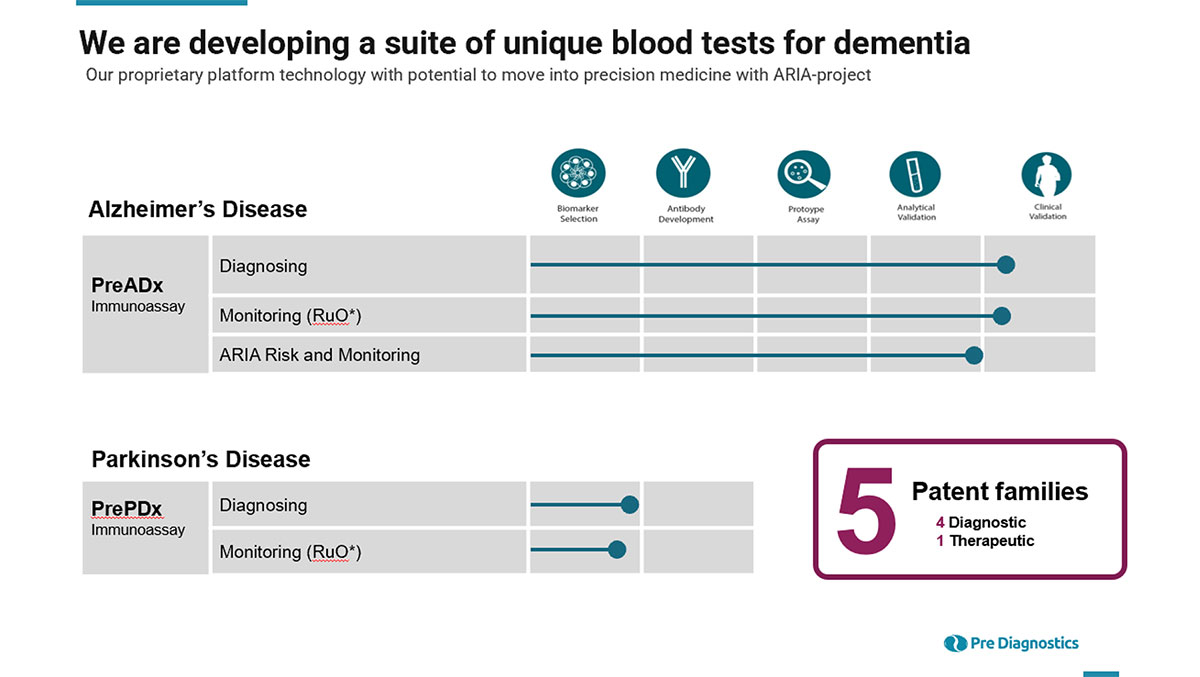
Pipeline
Our technology and the scientific premise it is founded on is relevant for several diseases, the company focus is currently on neurodegenerative diseases, where Alzheimer’s is first disease indication in the pipeline.
Pre ADx and Alzheimer’s disease
PreADx
PreADx is a dynamic test that measures the ongoing clearance of Abeta trough the detection of split products from the Abeta peptide. The first product, the X-34 abeta monocyte assay, has been CE marked, and is also ready for use as a Research Use Only product.
Alzheimer’s disease (AD) is a leading cause of dementia and poses a dramatic global health burden. More than 50 million people are currently living with dementia globally, with a projected increase to 152 million by 2050. The total worldwide cost of dementia is estimated to be US $1 trillion in 2019, and estimated to double by 20302. Although there are over 130 therapeutic candidates currently in pipeline, therapies under development have so far been failing. This in large part due to lack of understanding of the underlying disease mechanisms and the lack of optimal diagnostics tools that would enable for the necessary early diagnosis required for early intervention options. The recent FDA approval of Biogen’s immunotherapy and with four new immunotherapies with FDA Fast Track Designation, including Roche, Lilly and Eisai, focus is again moving towards early diagnosis and tools for early detection.
Our newest project entails the use of PreADx for personalized treatment of Alzheimer patient’s that are eligible for these new immunotherapies. The immunotherapies show promising clinical results, but between 10-40% of patients on high-dose treatment suffered from the serious adverse side event of “ARIA”, Amyloid-related imaging abnormalities, or brain hemmeraging. There is currently no available biomarker to detect patients at risk for ARIA, or monitor ongoing treatment for these patients.
PrePDx and Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disease affecting 1-2% of the population above 65 years of age. It is the second largest neurodegenerative disease, and the largest movement disorder. Upon diagnosis 60-70% of relevant brain neurons are affected leading to symptoms like shaking, stiffness and slow movements. PD can’t be cured, but medications might significantly improve the symptoms. Development of new medications are in pipeline, but rely heavily on the ability to detect PD at an early stage. Currently no such early, specific and definitive analytical test exists, and we aim to solve this challenge with our blood test.
The innate immune system plays a pivotal role in clearance of toxic proteins and is an essential feature of PD development. Our diagnostic concept, similar to AD, is based on the hypothesis that PD evokes unique systemic responses in immune cells, leading to poor protein clearance. Our test will quantify the clearance of specific protein called alpha-Synuclein, previously proven to have a central role in the destruction of brain neurons in PD patients. In a normal setting the protein will be cut into a number of smaller pieces as a part of the cell clearance. Since this cell machinery is not working optimally the protein will accumulate in specific immune cells of PD patients. Our test will quantitate specific fragments of alpha-synuclein that will enable detection and diagnosis of PD.
Pipeline Overview